Cleanroom-Operation Theater, Biosafety Lab, GMP Lab & Cleanroom
WHAT ARE WE OFFERING
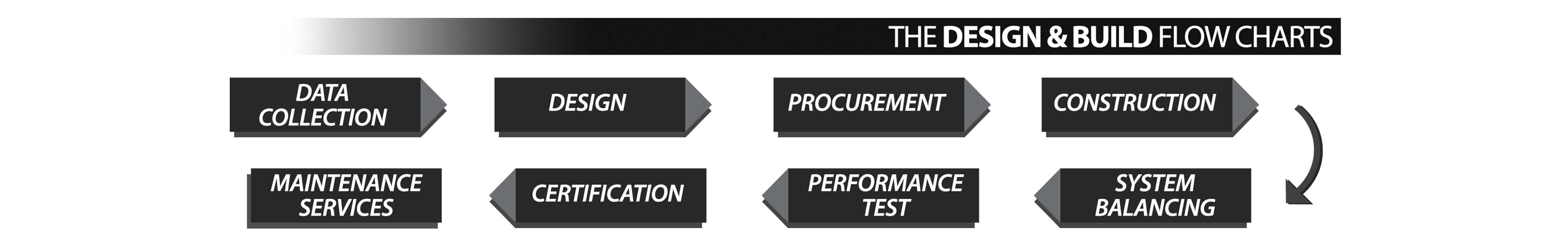
- Consultation, design & build the Critical Environment Facility
- Supply and Install Equipment for Laboratory, Operation Theatre & Cleanroom
- Testing and Balancing the System
- Certification of NEBB / ICS

OVERVIEW
A ‘clean room’ or a ‘clean space’ is defined as a room, a suite of rooms or an area within a controlled environment. The concentration of airborne particles in these areas is strictly controlled. Other factors may also be controlled within the limits of this space.
Clean spaces are used in manufacturing, packaging, laboratories and research. They are most often seen in the pharmaceutical, biotechnology, semiconductor, microelectronics and aerospace industries. Hospital theatres have a similar ‘clean space’, however here there is a need to control particular types of contamination, rather than the quantities of particles present.
We provides comprehensive Design & Built for Operation Theatre, Biosafety Lab, GMP Lab and Cleanroom which includes all the following :
1. Bio Cleanroom including Operation Theatre and Biohazard Rooms
2. Laboratories
3. Negative and Positive Pressure Isolation Rooms
4. Pharmaceutical Cleanrooms
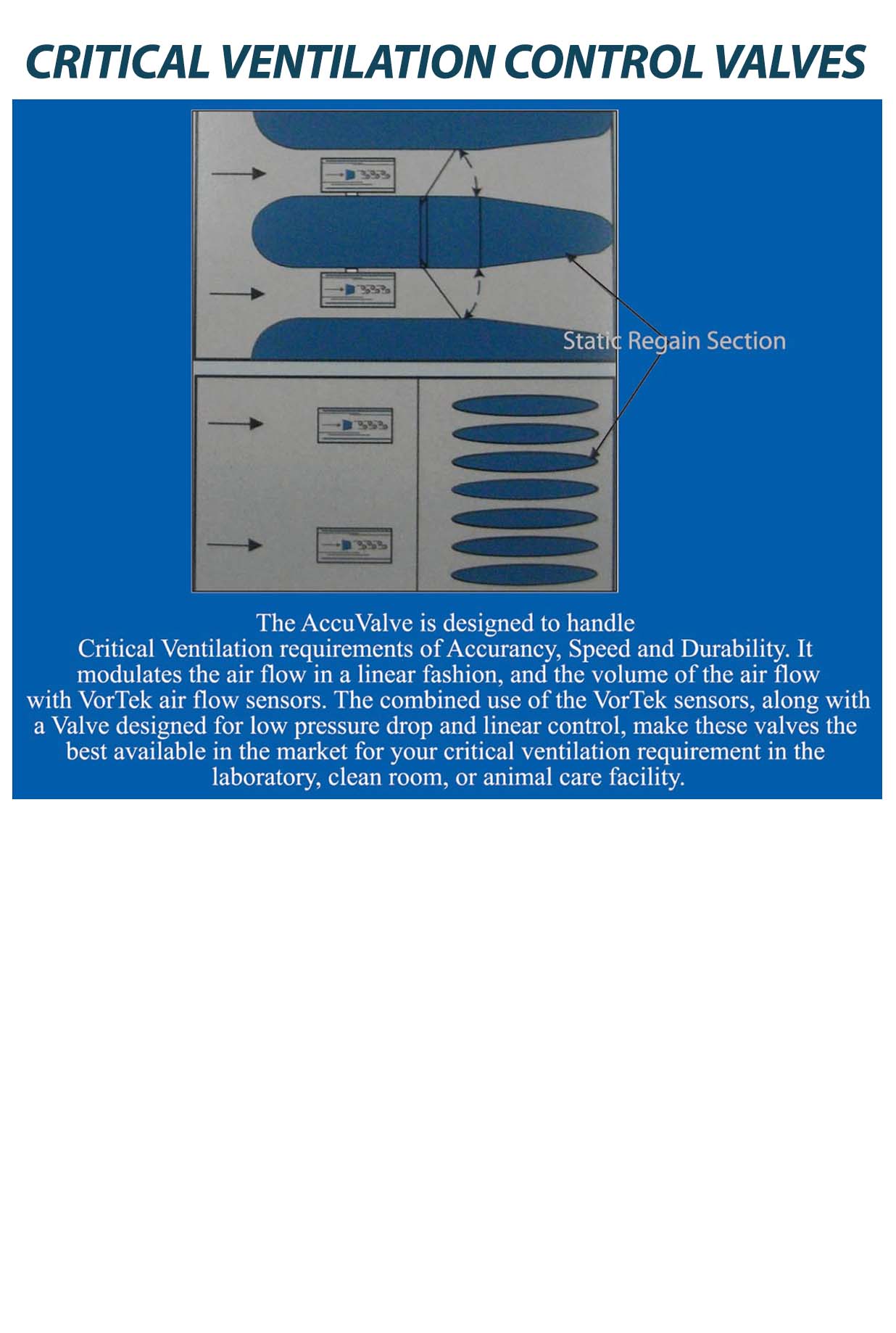
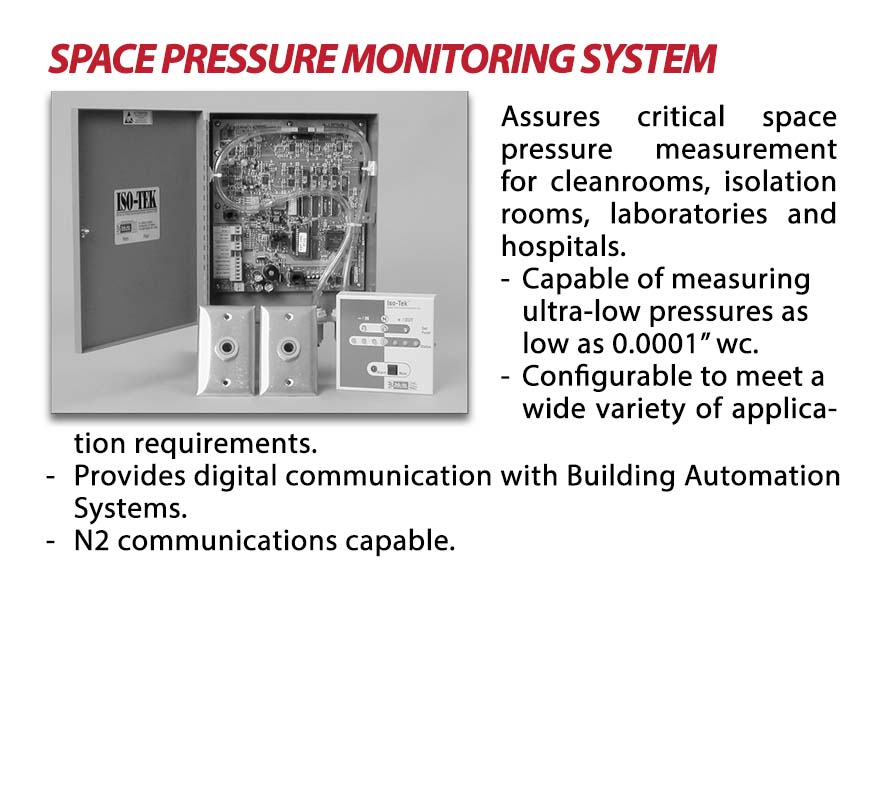
OUR CRITICAL ENVIRONMENT TESTING EQUIPMENTS
– Electric Balancing Tools with Air Flow Prohood
– Velometer
– Kistock Data Logger
– Minneapolis Door Blower
– Infrared Thermometer
– Air Sampling System
– Mini Incubator
– Particle Counter
– Tachometer

CRITICAL ENVIRONMENT
COMMON REQUIREMENTS
- Particle & Room Cleanliness
- Room Pressurization
- Airflow Velocity & Volume
- Temperature
- Humidity
CONTROL PARAMETERS
SPECIAL & UNIQUE REQUIREMENT
- Microbial Contamination
- Electrostatic Discharge (ESD)
- Gaseous Contamination
- Room Air Recovery
- Particle Fallout
- Lighting Level and Uniformity
- Sound Level
- Conductivity
- Electromagnetic Interference (EMI)
- Air Change Rate (ACH)
CRITICAL ENVIRONMENT BUILT WILL BE MADE TO COMPLY TO THE FOLLOWING STANDARDS
- BS EN ISO 14644-1
- FEDERAL STANDARD 209E
- BS 5295
- EC GMP ANNEX 1
- Limits of microbiological contaiment in clean areas
- PIC/S GMP for Medical Product
PROFESSIONAL MEMBERSHIP OF
- ASHRAE
- ASHRAE TC 9.11 Clean Spaces
- ASHRAE TC 9.6 Healthcare
- BIOMEDICAL ENGINEERING ASSOCIATION MALAYSIA (BEAM)
- Irish Cleanroom Society (ICS)
- International Society for Pharmaceutical Engineering (ISPE)
- Institute of Environmental Sciences and Technology (IEST)
OUR COLLABORATION
- Accutrol-Tek-Air, Inc (USA)
- Engsysco, Inc (USA)